|
SODIUM DICHLOROISOCYANURATE
| ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 2893-78-9 |
|
| EINECS NO. | 220-767-7 | |
| FORMULA | C3HCl2N3O3·Na | |
| MOL WT. | 219.95 | |
| H.S. CODE | 2933.69.6050 | |
|
TOXICITY |
Oral rat LD50: 1420 mg/kg | |
| SYNONYMS | 1,3-Dichloro-1,3,5-triazinetrione sodium salt; SDIC; Troclosene; | |
| Troclosene sodium; 1-Sodium-3,5-dichloro-1,3,5-triazine-2,4,6-trione; 1-Sodium-3,5-dichloro-s-triazine-2,4,6-trione; Sodium 1,3-dichloroisocyanurate; Simpla; Sodium salt of 1,3-dichloro-s-triazinetrione; 1,3-Dichloro-1,3,5-triazine-2,4,6(1H,3H,5H)-trione sodium salt; 1-Sodio-3,5-dichloro-s-triazine-2,4,6-trione; s-Triazine-2,4,6(1H,3H,5H)-trione, dichloro-, sodium salt; 1,3-Dichloro-s-triazine-2,4,6-(1H,3H,5H)-trione sodium salt; Monosodium 1,3-dichloroisocyanurate; troclosene sodium; Sodium dichloro-s-triazine trione; NaDCC; Other RN: 10119-30-9, 12676-23-2, 13023-28-4, 16499-74-4, 25717-18-4, 76560-28-6, 81918-50-5, 200401-83-8 | ||
|
SMILES |
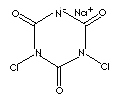
c1(n(c(nc(n1Cl)[O-])=O)Cl)=O.[Na+] | |
|
CLASSIFICATION |
Germicide, Bactericide, Disinfectant, Herbicide | |
|
EXTRA NOTES |
EPA Pesticide Chemical Code 081404 | |
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE | white crystalline granular or powder or Tablets | |
| MELTING POINT | 225 C | |
| BOILING POINT |
Decomposes ( 230 - 250 C) | |
| DENSITY | 0.7 | |
| SOLUBILITY IN WATER | 30g/100ml | |
|
SOLVENT SOLUBILITY |
0.5g/100ml in Acetone | |
| pH | ||
| VAPOR DENSITY | ||
| AUTOIGNITION |
| |
| NFPA RATINGS | Health: 2 Flammability: 0 Reactivity: 2 Physical hazards OX | |
| REFRACTIVE INDEX |
||
| FLASH POINT | 230 C | |
| STABILITY | Stable under ordinary conditions | |
|
EXTERNAL LINKS & GENERAL DESCRIPTION |
||
|
Drug Information Portal (U.S. National Library of Medicine) - Troclosene sodium PubChem Compound Summary - Sodium Dichloroisocyanurate http://www.ebi.ac.uk/chebi/ - Troclosene sodium http://www.ncbi.nlm.nih.gov/ - Sodium dichloroisocyanurate http://www.reproline.jhu.edu/ http://www.ncbi.nlm.nih.gov/ http://www.sigmaaldrich.com/ Local: Sodium Dichloroisocyanurate is a stabilised chlorine donor containing cyanuric acid and used as a disinfectant, industrial deodorant and biocide in water treatment. It is used in detergents as a sanitizers. |
||
|
SALES SPECIFICATION |
||
|
APPEARANCE |
white crystalline granular or powder or or Tablets | |
| CHLORINE CONTENT |
56.0% min or 60.0% min | |
| pH | 5.5 - 7.0 | |
| TRANSPORTATION | ||
| PACKING | ||
| HAZARD CLASS | Hazard Symbols: XN O N, | |
| UN NO. | 2465 | |
| SAFETY INFORMATION | ||
|
HAZARD OVERVIEW |
Oxidizer: Contact with combustible/organic material may cause fire. Risk of explosion by shock, friction, fire or other sources of ignition. Harmful if swallowed. Irritating to eyes, respiratory system and skin. Contact with acids liberates toxic gas. Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment. |
|
|
GHS |
|
|
| SIGNAL WORD |
Danger |
|
|
PICTOGRAMS |
|
|
|
HAZARD STATEMENTS |
H272: May intensify fire; oxidizer |
|
|
PRECAUTIONARY STATEMENTS |
P210: Keep away from heat/sparks/open flames/hot surfaces.
- No smoking |
|
| EC DIRECTIVES |
|
|
| HAZARD CODES |
|
|
|
RISK PHRASES |
2: Risk of explosion by shock, friction, fire or other
sources of ignition. |
|
|
SAFETY PHRASES |
2: Keep out of the reach of children. |
|
|
GENERAL DESCRIPTION OF CYANURIC ACID |
||
Cyanic acid
(the isomer of fulminic acid) is an unstable (explosive), poisonous, volatile,
clear liquid with the structure of H-O-C��N (the oxoacid formed from the
pseudohalogen cyanide), which is readily converted to
cyamelide and fulminic
acid. There is another isomeric cyanic acid with the
structure of H-N=C=O, called isocyanic acid. Cyanate group (and isocyanate group) can react with itself.
Cyanuric acid (also called pyrolithic acid), white monoclinic crystal with
the structure of [HOC(NCOH)2N], is the trimer
of cyanic acid. The
trimer
of isocyanic acid
is called biuret.
Cyanic acid hydrolyses to ammonia and carbon dioxide in water. The salts and esters of cyanic acid are cyanates. But esters of normal cyanic acid are not known. The salts and esters of isocyanic acid are isocyanates. The isocyanate group reacts with the hydroxyl functional group to form a urethane linkage. Diisocyanates (or polyisocyanates) are monomers for polyurethane production. Polyurethane is made from a variety of diisocyanates in conjunction with polyether and polyester polyols as co-reactants by addition polymerization which needs at least two -N=C=O groups. Polyurethanes are widely used in the manufacture of flexible and rigid foams, fibres, coatings, and elastomers. If isocyanate monomer is polymerized with amine group, polyurea is produced. Cyanates (or Isocyanates) are readily reacts with various form of amine (including ammonia, primary-, secondary-amines, amides and ureas) and hydroxyl functional group. They are used in the synthesis for the target molecules such as pharmaceuticals, pesticides, textile softener, lubricants and industrial disinfectants. They can convert to polycyclic compounds such as hydantoins and imidazolons. They are used as plastic additives and as heat treatment salt formulations for metals. |
||
|
PRICE INFORMATION |
||